Procedural Requirements for Initiating Clinical Trials in The European Union
Prior to initiation of a first-in-human clinical trial, a sponsor must submit scientific data to demonstrate that the drug product is safe for use in humans. The scientific documentation requirements are similar for the European Union (E.U.) and the United States (U.S.). The Common Technical Document (CTD) format, as described in International Committee for Harmonization (ICH)M4Q1, is used as a guide for the content development for both Investigational New Drug Application (IND) and Investigational Medicinal Product Dossier (IMPD) submissions to the health authorities in the U.S. and E.U., respectively.
However, additional procedural requirements are in place specifically for clinical trial initiation in the E.U. These E.U.-specific procedural requirements will be discussed in this article, and when relevant, compared to U.S.-specific requirements.
Good Manufacturing Practice (GMP) certificates
Both the E.U. and the U.S. require that the clinical trial product is manufactured under GMP conditions. In the U.S., drug manufacturers must comply with the current GMP (cGMP) regulations to assure product quality and safety2. The highly trained U.S. Food and Drug Administration (FDA) employees inspect the pharmaceutical manufacturing facilities based on a standard approach to assure compliance with cGMP regulations. The FDA also uses reports of potentially defective drug products from the public and the industry for recognition of sites that need to be inspected or investigated3.
The E.U. requires that each supply chain site has a GMP certificate to manufacture investigational medicinal products. A GMP certificate can only be issued following a GMP inspection by a national competent authority4. This certificate confirms the GMP compliance status of the manufacturing site. The site inspection can occur a spart of a product-specific inspection and/or based on a general GMP inspection. Regardless of the inspection objective, if the site is operated in accordance with GMP requirements, a site-specific (not product specific) GMP certificate is issued. However, depending on the scope of the inspection and the inspection results, the GMP certificate can be restricted to specific activities within a given site. Therefore, it is critical to confirm that the site that is chosen for manufacturing and testing of a drug product intended for use in clinical trials in the E.U. has a GMP certificate5. The national competent authorities must enter the GMP certificates into the EudraGMDP database. EudraGMDP is a Union database that is publicly available and all GMP certificates and non-compliance reports are deposited there. It should be noted that any GMP certificate in the EudraGMDP is mutually recognized, and the database authenticates the certificate4.
Qualified Person (QP) Certification
The usage of investigational medicinal products (and approved products) in the E.U. requires that the product is certified by a QP6. The QP certifies that each individual batch has been manufactured in compliance with the laws that are enforced in the Member State (MS) where certification takes place, in conformity with the requirements of the IMPD and with GMP7. The QP declaration cannot be issued until after the clinical trial product has been manufactured and the IMPD is approved by the MS. However, a suitable QP should be identified 6 to 9 months prior to submission of a clinical trial application to support planning for QP activities.
The QP should have access to all documents related to the supply chain of the active substance and medicinal product. This will allow the QP to schedule GMP audits or to evaluate prior QP declarations for a specific site. Finally, the early selection of a suitable QP will allow development of the Product Specification File (PSF), which includes all documentation, needed to support the QP certification.
IMPs are released in a two-step process in which the QP certifies the batch per the above-mentioned procedures and the sponsor provides the regulatory release (e.g., provides assurance that appropriate contracts are in place, regulatory authorization has been provided by the national competent authority and that any regulatory conditions are fulfilled).
Import license
Sponsors that manufacture IMPs outside the E.U. are required to obtain an import license for importation of the IMP into the E.U. An import license is granted by the regulatory authorities of the member state in which the importing activity takes place. All importing licenses are deposited in EudraGMDP8. The importer verifies that the transportation and storage conditions are done in a suitable manner, hence maintaining product quality during shipment. Further, during shipment of the IMP to the clinical investigator site, the applicable guidelines on Good Distribution Practice (GDP) should be taken into consideration by all accountable parties to minimize any risk relating to quality of the product7. The respective responsibilities of the sponsor, manufacturer, importer and, where used, distributor should be determined in a technical agreement9. It is noteworthy to mention that the ultimate responsibility is with the sponsor5. The sponsor oversees the entire chain of distribution of IMP from manufacture or importation into the E.U., through supply to the investigator sites. This exercise ensures that IMP is stored, transported, and handled under controlled condition.
IMPD submission and review procedures
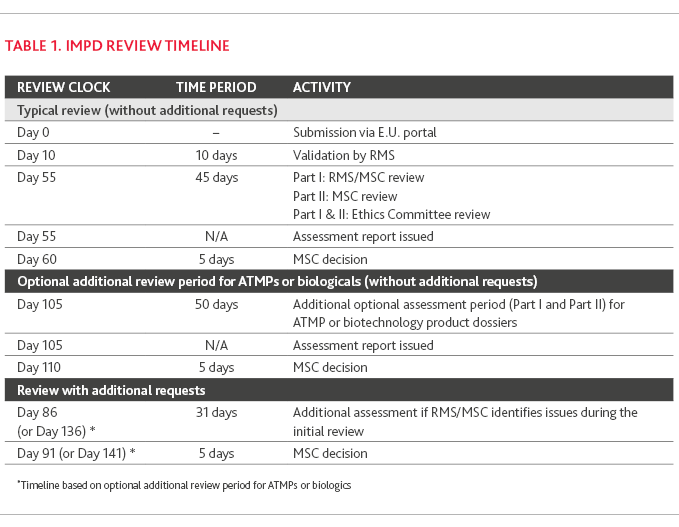
The initial regulatory submission and review in the E.U. has several steps. In order to obtain an authorization, the sponsor should submit the application dossier via the E.U. portal, using a harmonized format, to all Member States Concerned (MSC) where the clinical trial will be conducted10. The harmonized format consists of two parts. Part I contains the common scientific documents including IMPD and part II contains the national documents. The sponsor should propose one of the MSCs as the Reporting Member State (RMS) that will coordinate validation and evaluation of the application. In general, part I and II are submitted together but it can be submitted separately. If submitted separately, part II must be submitted within 2 years of part I11. The RMS considers comments issued by other MSCs and validates the submitted dossier within 10 days. After the validation step, assessment of the application will be conducted for part I and II. The scientific part (part I) is reviewed by the RMS.
For a multinational trial, all MSCs collaborate in this assessment. Part II is typically reviewed in parallel with part I by the MSCs. The Ethic Committee (EC) performs the ethical review of part I and part II, as needed, in accordance with the law of the MSC. Within 45 days from validation date, the RMS consolidates all comments and issues a part I assessment report. For Advanced Therapy Medicinal Products (ATMPs) or biotechnology medicinal products, the initial assessment period may be extended by an additional 50 days. If an issue was raised within the initial assessment period, the RMS may ask the sponsor for additional information. In this case, the assessment period is extended with an additional 31 days11 which includes 12 days for the sponsor to respond, 12 days for review and 7 days for consolidation of MS review. The timeline for final decision of the MSC is within 5 days of the part I evaluation reporting date or by the last day of the part II evaluation. The sponsor will be notified through the E.U.-portal by the MSC(s).
The timelines for IMPD review process are summarized in Table 1. IMPD Review Timeline.
Clinical trial regulation
Starting 31 January 2022, the E.U. introduced the Clinical Trial Regulation (Regulation (E.U.) No.536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicines for human use12) which allows sponsors to submit a single application for multinational trials via the Clinical Trial Information System (CTIS)13. This new system provides an opportunity for the sponsors to run a clinical trial in several European countries and conduct multinational trials in a more effective path. It also allows the MSCs to evaluate and authorize applications collaboratively.
The CTIS is being introduced in a stepwise manner, in line with a three-year transition period and hence new and current applications can remain under the Clinical Trial Directive ((EC) No. 2001/20/EC)14.
Parallel Scientific Advice (PSA) during early development of IMPs
Finally, as medicines development moves towards a globalized approach, both the pharmaceutical industry and regulatory agencies are looking for opportunities to collaborate early in product development. To fulfill this goal, the PSA program shared by the European Medicines Agency (EMA) and FDA provides a mechanism for evaluators and reviewers to concurrently engage in scientific issues with sponsors on key subjects during the development phase of new medicinal products15. PSA procedures are voluntary and are based on the sponsor request to focus on sharing information and perspectives. Both agencies follow domestic procedure and review goals and timelines.
References
-
https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m-4-q-common-technical-document-registration-pharmaceuticals-human-use- quality-step-5_en.pdf
-
European Medicines Agency
-
European Medicines Agency
-
European Medicines Agency
-
European Medicines Agency
-
European Medicines Agency
-
https://www.cromsource.com/wp-content/uploads/2015/02/The-EU-Clinical-Trials-Regulation-Main-Changes-and-Challenges.pdf
-
https://ec.europa.eu/health/system/files/2016-11/reg_2014_536_en_0.pdf
-
https://health.ec.europa.eu/medicinal-products/clinical-trials/clinical-trials-directive-200120ec_en
-
https://www.fda.gov/media/105211/download
SHARE